Certification Requirements and Process for Exporting Medical Devices to the EU
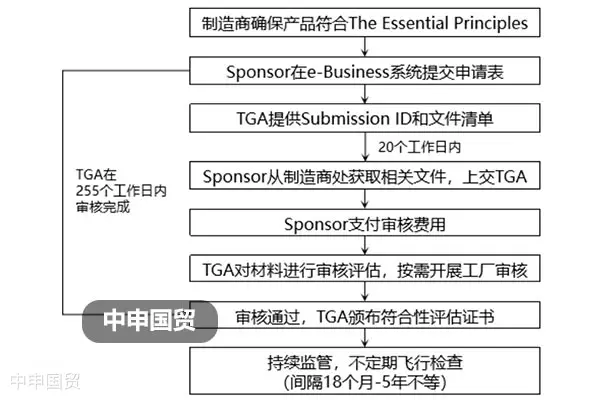
The medical device industry is a vital sector globally, covering a wide range of medical fields including diagnosis, treatment, and rehabilitation. With the advancement of globalization, the export trade of medical devices has become increasingly frequent. The EU, as one of the worlds largest economies, has a huge demand for medical devices. However, exporting medical devices to the EU requires compliance with a series of certification requirements, which is a significant task for exporters.











 PSB Record: Shanghai No.31011502009912
PSB Record: Shanghai No.31011502009912



