- Shanghai Zhongshen International Trade Co., Ltd. - Two decades of trade agency expertise.
- Service Hotline: 139 1787 2118
In recent years, BrazilsMedical Equipmentindustry has continued to develop and has become the largest medical device market in Latin America. For exporters, understanding Brazils medical device access regulations is crucial. This article provides an in-depth analysis of Brazils medical device legislative bodies, medical device classification, and market access for medical device products.
Brazils Medical Device Legislation and Regulatory Authorities
The fundamental regulation for medical device supervision in Brazil is RDC185/01. This regulation is enforced by the National Health Surveillance Agency (ANVISA). ANVISA is Brazils primary regulatory body, responsible for approving and supervising market access for medical products.
Classification of Medical Devices in Brazil
In Brazil, medical devices are classified into four risk levels: Class I, II, III, and IV. Class I represents the lowest risk, while Class IV represents the highest risk. The classification rules for medical devices are detailed in Appendix II of the RDC185 regulation.
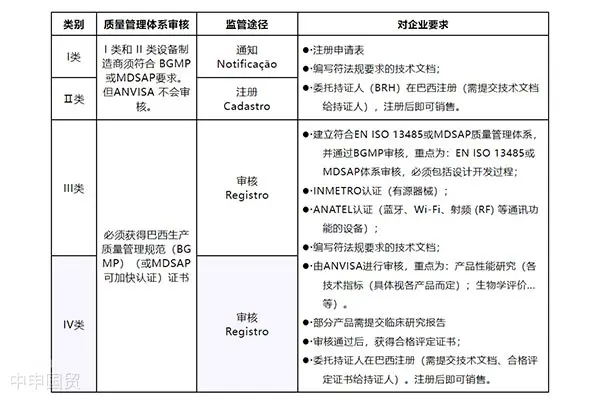
Market Access for Medical Device Products in Brazil
Brazil has strict regulations for any products involving the human body (including pharmaceuticals, medical devices, cosmetics,Cosmetics & Personal Careetc.). Before selling these products, exporters must submit a written application to the Brazilian Ministry of Health and provide registration materials through officially designated registration holders. These materials must primarily be in Portuguese.
Medical device products with power sources require certification. The certification process is conducted by third-party agencies, which will test the products and audit the manufacturing facilities. For higher-risk Class III and IV medical devices, manufacturers can opt for the MDSAP (Medical Device Single Audit Program) audit as an alternative to ANVISAs audit. Notably, Brazil has currently authorized 11 MDSAP testing agencies.
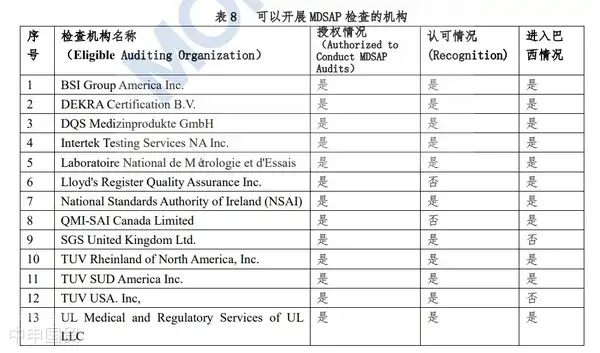
(1) What is MDSAP
MDSAP stands for Medical Device Single Audit Program, initiated by the MDSAP Regulatory Authority Committee of the International Medical Device Regulators Forum (IMDRF). MDSAP, commonly known as the five-nation joint audit, is a new audit program recognized and joined by regulatory authorities from five countries: the United States (FDA), Australia (TGA), Brazil (ANVISA), Canada (HC), and Japan (MHLW).
(2) ANVISA Registration Process
For non-local manufacturers looking to sell medical devices in the Brazilian market, certification and registration must be completed in accordance with ANVISA regulations. The basic steps and processes include determining product categories, appointing a Brazilian Registration Holder (BRH), obtaining INMETRO certification, undergoing BGMP factory inspections, preparing technical documentation, paying application fees, and ultimately obtaining approval for sales.
(3) Main regulations applicable to the market authorization of exported medical devices in Brazil
In Brazil, market authorization for medical devices must comply with several key regulations, including the access regulations RDC 185/2001 and RDC 40/2015 for medical devices, as well as the clinical trial regulations RDC 10/2015 and the GMP regulations RDC 183/2017 for medical device manufacturers.
Although the Brazilian medical device market is vast, the market access regulations are relatively complex. For exporters looking to sell products in the Brazilian market, understanding and complying with these regulations is crucial. This not only ensures their products can be launched compliantly but also helps reduce potential legal risks and unnecessary costs.
Related Recommendations
Category case
Contact Us
Email: service@sh-zhongshen.com
Related Recommendations
Contact via WeChat

? 2025. All Rights Reserved. 滬ICP備2023007705號-2  PSB Record: Shanghai No.31011502009912
PSB Record: Shanghai No.31011502009912